Broad Center for Stem Cell Research and Jonsson Comprehensive Cancer Center
TheChuteLab
Research
Discovery of Novel Mechanistic Targets for Therapeutic Hematopoietic Regeneration
Total body irradiation (TBI) and myeloablative chemotherapy are successfully utilized in the conditioning of patients for hematopoietic cell transplantation. Ionizing Radiation (IR) causes toxicity to hematopoietic stem cells (HSCs) through the generation of reactive oxygen species, DNA strand breaks, induction of apoptosis and damage to the bone marrow (BM) microenvironment. Despite the understanding of mechanisms through which IR causes hematopoietic toxicity, few effective mitigators of radiation-induced hematopoietic injury have been developed. Elucidation of novel mechanisms through which HSCs respond to radiation and the development of therapeutics targeting such mechanisms could potentially benefit both victims of Acute Radiation Sickness as well patients receiving TBI for hematopoietic cell transplantation.
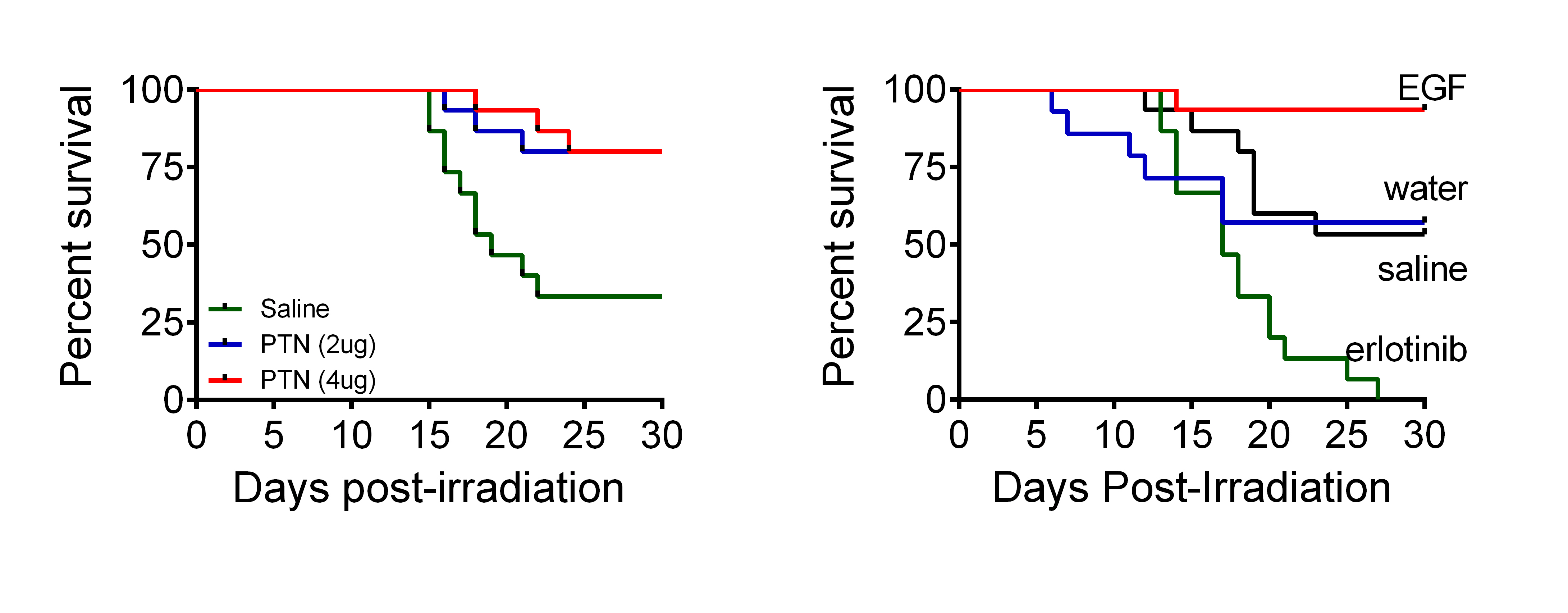
HSCs reside in specialized niches within the BM and distinct cells within these niches regulate HSC maintenance in vivo. We have shown that BM ECs regulate the response of HSCs to genotoxic stressors such as IR (1-3). However, the precise mechanisms through which BM niche cells promote HSC regeneration after injury remain poorly understood. We recently described the hematopoietic function of PTN, a protein which is secreted by BM ECs and which promotes the expansion of long-term HSCs (LT-HSCs) in culture (4). Deletion of PTN in the BM microenvironment significantly decreased LT-HSCs in mice (4). Furthermore, we recently demonstrated that systemic administration of PTN increased the survival of lethally irradiated mice via induction of Ras/MEK/ERK signaling in HSCs and by promotion of HSC quiescence after irradiation (5). In parallel studies, we also recently showed that hematopoietic stem cells upregulate the expression of epidermal growth factor receptor (EGFR) in response to ionizing radiation and that BM ECs secrete EGF in the HSC niche, leading to HSC regeneration in vivo after radiation injury (6). Importantly, we showed further that EGF mediates these effects via repression of PUMA-mediated cell death in HSCs and systemic administration of EGF substantially increased mouse survival following lethal dose irradiation (6). Currently, we are studying the role of other cells within the HSC niche, specifically mesenchymal progenitor cells and osteoblasts, to determine whether additional cell types in the HSC niche also regulate HSC regeneration after injury and the mechanisms through which HSC regeneration is triggered by the microenvironment.
These studies are fundamentally important to defining the mechanisms governing HSC regeneration and also will provide the mechanistic basis for the development of therapeutics to promote human hematologic reconstitution in patients following myelosuppressive chemo/radiotherapy for cancer or following acute radiation sickness.
1. Doan P, et al. Tie2(+) bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury. Stem Cells. 2013;31(2):327-337.
2. Salter, A.B., et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113(9):2104-7.
3. Chute JP, et al. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood.2007;109:2365-2372.
4. Himburg, H.A., et al., Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nature Medicine. 2010;16(4):475-82.
5. Himburg, H.A., et al., Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche.Cell Reports. 2012;2(4):964-75.
6. Doan PL, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nature Medicine. 2013;19(3):295-304.
Total body irradiation (TBI) and myeloablative chemotherapy are successfully utilized in the conditioning of patients for hematopoietic cell transplantation. Ionizing Radiation (IR) causes toxicity to hematopoietic stem cells (HSCs) through the generation of reactive oxygen species, DNA strand breaks, induction of apoptosis and damage to the bone marrow (BM) microenvironment. Despite the understanding of mechanisms through which IR causes hematopoietic toxicity, few effective mitigators of radiation-induced hematopoietic injury have been developed. Elucidation of novel mechanisms through which HSCs respond to radiation and the development of therapeutics targeting such mechanisms could potentially benefit both victims of Acute Radiation Sickness as well patients receiving TBI for hematopoietic cell transplantation.

HSCs reside in specialized niches within the BM and distinct cells within these niches regulate HSC maintenance in vivo. We have shown that BM ECs regulate the response of HSCs to genotoxic stressors such as IR (1-3). However, the precise mechanisms through which BM niche cells promote HSC regeneration after injury remain poorly understood. We recently described the hematopoietic function of PTN, a protein which is secreted by BM ECs and which promotes the expansion of long-term HSCs (LT-HSCs) in culture (4). Deletion of PTN in the BM microenvironment significantly decreased LT-HSCs in mice (4). Furthermore, we recently demonstrated that systemic administration of PTN increased the survival of lethally irradiated mice via induction of Ras/MEK/ERK signaling in HSCs and by promotion of HSC quiescence after irradiation (5). In parallel studies, we also recently showed that hematopoietic stem cells upregulate the expression of epidermal growth factor receptor (EGFR) in response to ionizing radiation and that BM ECs secrete EGF in the HSC niche, leading to HSC regeneration in vivo after radiation injury (6). Importantly, we showed further that EGF mediates these effects via repression of PUMA-mediated cell death in HSCs and systemic administration of EGF substantially increased mouse survival following lethal dose irradiation (6). Currently, we are studying the role of other cells within the HSC niche, specifically mesenchymal progenitor cells and osteoblasts, to determine whether additional cell types in the HSC niche also regulate HSC regeneration after injury and the mechanisms through which HSC regeneration is triggered by the microenvironment.
These studies are fundamentally important to defining the mechanisms governing HSC regeneration and also will provide the mechanistic basis for the development of therapeutics to promote human hematologic reconstitution in patients following myelosuppressive chemo/radiotherapy for cancer or following acute radiation sickness.
1. Doan P, et al. Tie2(+) bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury. Stem Cells. 2013;31(2):327-337.
2. Salter, A.B., et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113(9):2104-7.
3. Chute JP, et al. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood.2007;109:2365-2372.
4. Himburg, H.A., et al., Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nature Medicine. 2010;16(4):475-82.
5. Himburg, H.A., et al., Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche.Cell Reports. 2012;2(4):964-75.
6. Doan PL, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nature Medicine. 2013;19(3):295-304.
